Within the Complexurf laboratory, one of the projects that our research team is actively engaged in is focused on advancing the development of novel surface-based strategies for anti-icing applications.
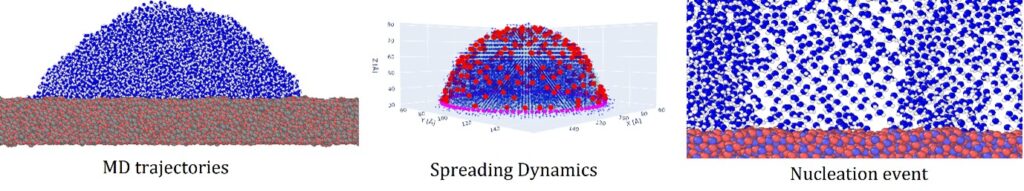
Within this context, our research endeavors are driven by a modeling approach to provide a better understanding of the intricate physics of icing phenomena. Our nanoscale modeling of ice nucleation begins with the deposition of supercooled water nanodroplets on cold solid substrates. A first characterization of the liquid state can be leveraged into the design of new surfaces with anti-icing properties. Additionally, by taking advantage of the atomistic-scale resolution is possible to obtain valuable information from the spreading dynamics that translate into a better understanding of the heterogeneous ice nucleation at the nanoscale level. Moreover, through the use of molecular simulation is possible to shed light on the electro-freezing phenomenon, a peculiar event observable only through the lens of the nanoscale. Furthermore, through conformational analysis of the lattice that arises from the supercooled water, we aim to determine new parameters that can be implemented into a new classical nucleation theory for water molecules at the nanoscale level. These advancements in classical nucleation theory will not only enhance our understanding of the nucleation process but also contribute to the development of more accurate models for ice formation. The findings from our research will provide insights into the design of ice-phobic materials with enhanced anti-icing properties. By gaining a better understanding of the nucleation process of supercooled droplets, we can rationalize and optimize the design of surfaces and coatings that effectively repel ice formation.
In conclusion, our nanoscale modeling of ice nucleation research within the Complexurf laboratory contributes to the advancement of surface-based strategies for anti-icing applications. The understandings gained from our modeling approach enable us to design novel ice-phobic materials, improve classical nucleation theory, and address the challenges associated with ice formation in various industries.